Ultrapure Water System Testing|Panel & Standard Resistivity Verification
Ultrapure Water System Testing|Panel & Standard Resistivity Verification|READY Biotech
In biomedical, analytical chemistry, and research labs, having stable and accurate ultrapure water is fundamental for reproducibility and data integrity. If a system’s panel display does not reflect actual water quality or resistivity drops below the threshold, it can lead to reagent contamination, analytical errors, or experimental failure. READY Biotech offers a thorough testing/calibration service focusing specifically on resistivity verification and panel-to-standard comparison.
▣ Testing Items & Detailed Procedures
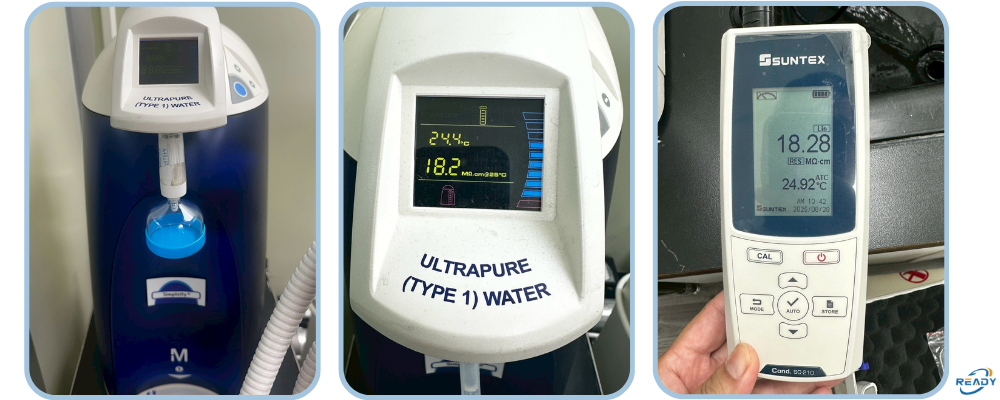
☑ Resistivity Testing – Measurement of actual output water resistivity using a calibrated resistivity meter (Resistivity Meter) at the ultrapure water outlet or sampling point.
☑ Panel / Standard Piece Comparison – Compare the system’s panel display reading with a standard piece/standard resistivity value to check whether the display error falls within acceptable tolerance.
☑ Threshold Standard – The benchmark resistivity is ≥18.2 MΩ·cm (25°C), following international ultrapure water quality norms.
☑ Instrument and Standard Piece – The resistivity meter and standard piece used are both certified/calibrated by recognized calibration labs (TAF or equivalent).
☑ Test Report – After completion, you receive a detailed report showing comparison data: panel readouts vs standard piece readings & measured resistivity values. (No curve graph is produced.)
Purpose & Value of the Test
The aim of this verification test is more than just ensuring that the display looks correct—it ensures that the actual purified water meets high purity standards required for experiments or production. Through this kind of testing and comparison, clients can:
-
Detect filter degradation, ion exchange or membrane performance issues early
-
Prevent major losses or sample rejections in research / pharmaceutical / analytical processes due to unstable water purity
-
Provide clear documentation for GLP, GMP, or ISO audits
-
Build trust in equipment performance and water quality among lab staff or manufacturing partners
Contact READY Biotech for professional verification and compliance documentation.
Contact Us
☎︎ TEL:+886-2-8228-0131
✉︎ EMAIL:Ready_lab@outlook.com